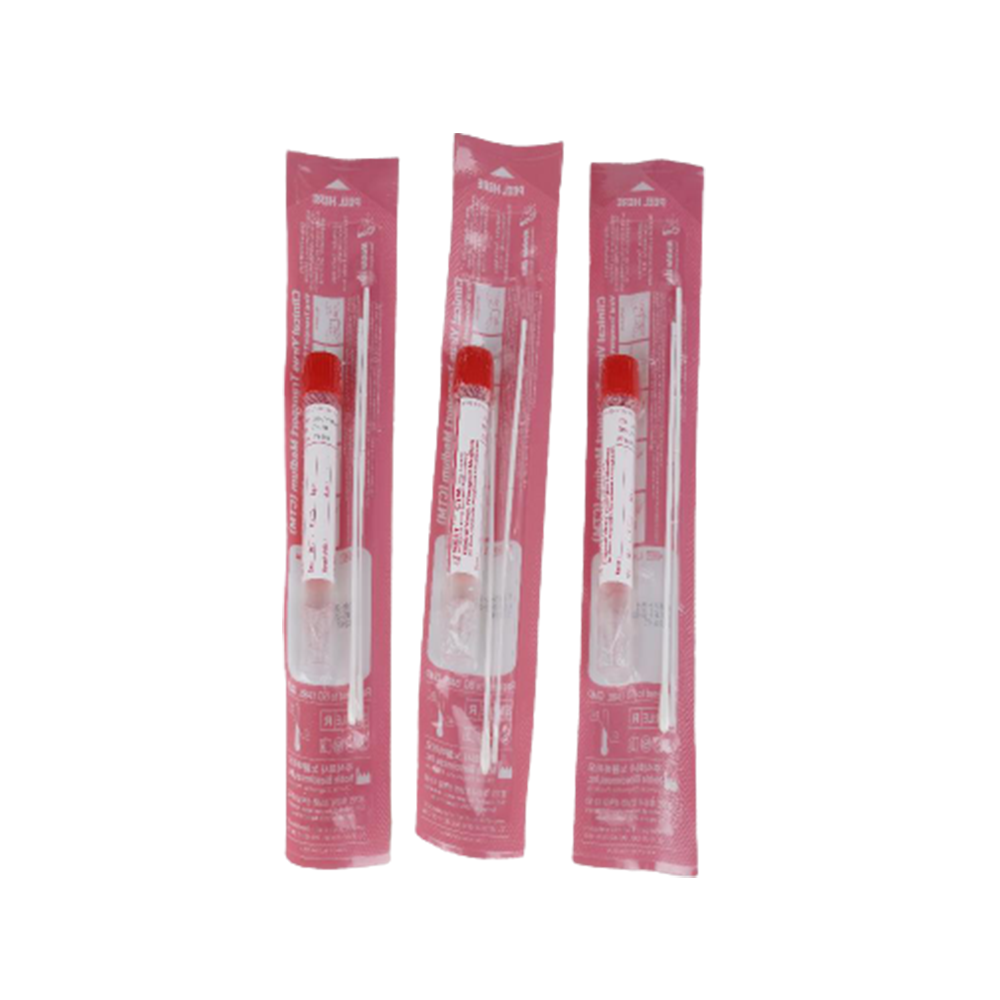
Clinical Virus Transport Medium
-
Payment
CAD , L/C(sight) , T/T
-
MOQ
10,000 Unit
-
Supply Details
Price is determined after consultation and consultation
-
Country of sale
World Wide, Asia, Americas, Europe, Middle East
-
PRICE
-
EXW
USD 1.40
(1 Unit)
-
ITEM SPECIFICS
-
Brand
Model CTMClinical Virus Transport Medium
-
origin
Republic of Korea
-
Size(Capacity)
Total length 150.8mm
-
Material
Tube(PP), Media(CTM media), Swab(POM or PP)
-
Condition
new
PRODUCT DESCRIPTION
T-SWAB TRANSPORT' Clinical Virus Transport Medium (CTM)
Viral Transport for Virus, Chlamydia, Mycoplasma & Ureaplasma Noble Biosciences Clinical Virus Transport Medium (CTM) is
Viral Transport for Virus, Chlamydia, Mycoplasma & Ureaplasma Noble Biosciences Clinical Virus Transport Medium (CTM) is
intended for the collection and transport of clinical specimens containing Virus, Chlamydia, Mycoplasma and Ureaplasma from the collection site.
The test procedures for quality control are based upon the quality control methods described in CLSI M40-A2 and others.
CTM transport medium is stable for 24 months at room temperature. Specimens in CTM medium are stable for 48 hours at room temperature.
This CTM collection and transport system is certified as Biopsy Procedure Kit. class II with product-license number 15-211 by the Korean FDA.
Application
- All viruses (Influenza A and B, Herpes Simplex, I and II, Cytomegalovirus, Respirato
- Chlamydia tachomatis and pneumoniae
- Mycoplasma hominis and pneumoniae
- Urea plasma urealyticum
- Enzyme Immuno Assays (EIA)
Clinical Virus Transport Medium (CTM)
T-SWAB TRANSPORT" CTM
113 CTM medium transport specimens in safety by using self-standing conical El There are various swab applicators for
the sample collection 13 Specimens in CTM are stable for 48 hours at room temperature Glass beads in CTM can discharge specimens from the swab by
PAYMENTS DETAILS
This supplier supports payments for offline orders
- Letter of Credit : L/C(sight)
- Cash Against Document : CAD
- Telegraphic Transfer : T/T
- Name : Charley Jung
SHIPPING
Shipping from :
Republic of Korea
- 13-50 Sinbaek-gil Jeongnam-myeon, Hwaseong-si, Gyeonggi-do (18521)
Noble Bio Co.Ltd
The person in charge
Gye-sueng BaekAddress
13-50 Sinbaek-gil Jeongnam-myeon, Hwaseong-si, Gyeonggi-do (18521)
Introduction
Our company is growing rapidly in line with the rapidly changing market trend with the development of eco-friendly and innovative technologies in the field of diagnostic reagents and life sciences. As an in vitro diagnostic (IVD) business field, we develop and produce swabs, transport media, and functional tubes that collect, store, and transport disease-causing specimens such as microorganisms and viruses in the surrounding environment and human body. ) We are jointly developing and manufacturing related parts by fusion with the results of our customers' in-depth efforts. In the field of biotechnology, we have secured a number of intellectual property rights by developing cell culture media, nucleic acid purification kits, research tubes, and tips.
We will always do our best to satisfy our customers, and we look forward to your continued interest and support.
-
- Business Type :
- Manufacturer
-
- Main Product :
- Collection Swab
-
- Established :
- 2008-07-01
-
- Total Annual Revenue :
- 4~5 billion (KRW)
-
- Total Employees :
- 11~50 people
Please suggest a variety of your ideas such as design, impact, enhancements, etc
Captcha Required
Please enter the text on the left image to prevent automatic input.
0 / 4000
질문이 없습니다.
CUSTOMER REVIEWS (0)
TRADE EXPERIENCE
-
- Total revenue
- 4~5 billion (KRW)
-
- Total export revenue (previous year in USD)
- 0
-
- Number of foreign trade employees
- 11~50 people
COMPARISON TO SIMILAR ITEMS more
- No Items
- supplier level
- MEMBER
- Noble Bio Co.Ltd
- Seller's Store url
- Response Level
★ ★ ★ ★ ★

- Supplier Level
★ ★ ★ ★ ★

- Transaction Level
★ ★ ★ ★ ★

SUPPLIER BEST
- No Items